Immunotherapy in Research and Treatment
Aline, when one thinks about immunotherapy, cancer comes to mind, but immunotherapy is used much more widely. Aside from cancer, where do you see its most potential?
For cancer applications, immunotherapies are used to encourage the patient's own cells to attack the cancer, and when I think about the initial skepticism towards cancer immunotherapies a century ago, the fact that we have not one, but several "cancer vaccines" approved for patient use is an amazing feat.
Still, most approved immunotherapies attempt to do the opposite, that is discourage a patient's cells from attacking. Sometimes immune cells attack what they shouldn't. Slowing down autoimmune diseases like multiple sclerosis and type-1 diabetes is the first application to come to mind.
Other times, immune cells overreact. Anti-inflammatories and immunosuppressants have been used to alleviate and inhibit the cytokine storms responsible for many of the severe complications of infections like COVID-19 and traumas such as spinal cord injury.
And sometimes, we want to trick immune cells to ignore, or better yet accept, a beneficial therapy--you may recognize immunosuppressants most for their role in enabling cell and organ transplantation. I should also mention that resistance to drugs used long-term for treatment can also have an immune component and quite a bit of research tries to design these drugs to avoid immune cell detection.
Although immunotherapy has existed for more than a century, there have been great developments in the past years. Could you name some of the milestones?
If herbal remedies with natural anti-inflammatory and immune cell-modifying properties like green tea are considered, one could argue immunotherapies have been around for millennia. In the 1800's, we started making real progress isolating and identifying the active ingredients in these treatments, leading to the first-generation of modern-day immunomodulating drugs. Soon after, pharmaceutical companies we recognize today started to appear and they developed ways to chemically modify natural compounds to improve their potency and decrease their side-effects, the most notable one being Aspirin in 1897. Around the same time (1891) was also the first report of a cancer vaccine that used inactivated bacteria to stimulate immune cells to attack the tumor. Subsequent drugs and therapies were more specific in their mode of action, but I would have to say the next major milestone was the development of immunosuppressive drugs to slow down transplant rejection in the 1960's. In later years many of these drugs were found to be effective in patients with autoimmune diseases as well. The first monoclonal antibody approved by the FDA for this purpose, Orthoclone OKT3, was in 1986. Also, though cell-based therapies were attempted early on, and were successful in preclinical models for a wide range of diseases, only recently have they been approved for clinical use. Sipuleucel-T, wherein the patient's own immune cells are modified to attack the cancer cells, was approved slightly over a decade ago. And last but not least, the first two engineered immune cells (car-T therapies) were approved for cancer in 2017.
What is the role that imaging plays in immunotherapy treatment?
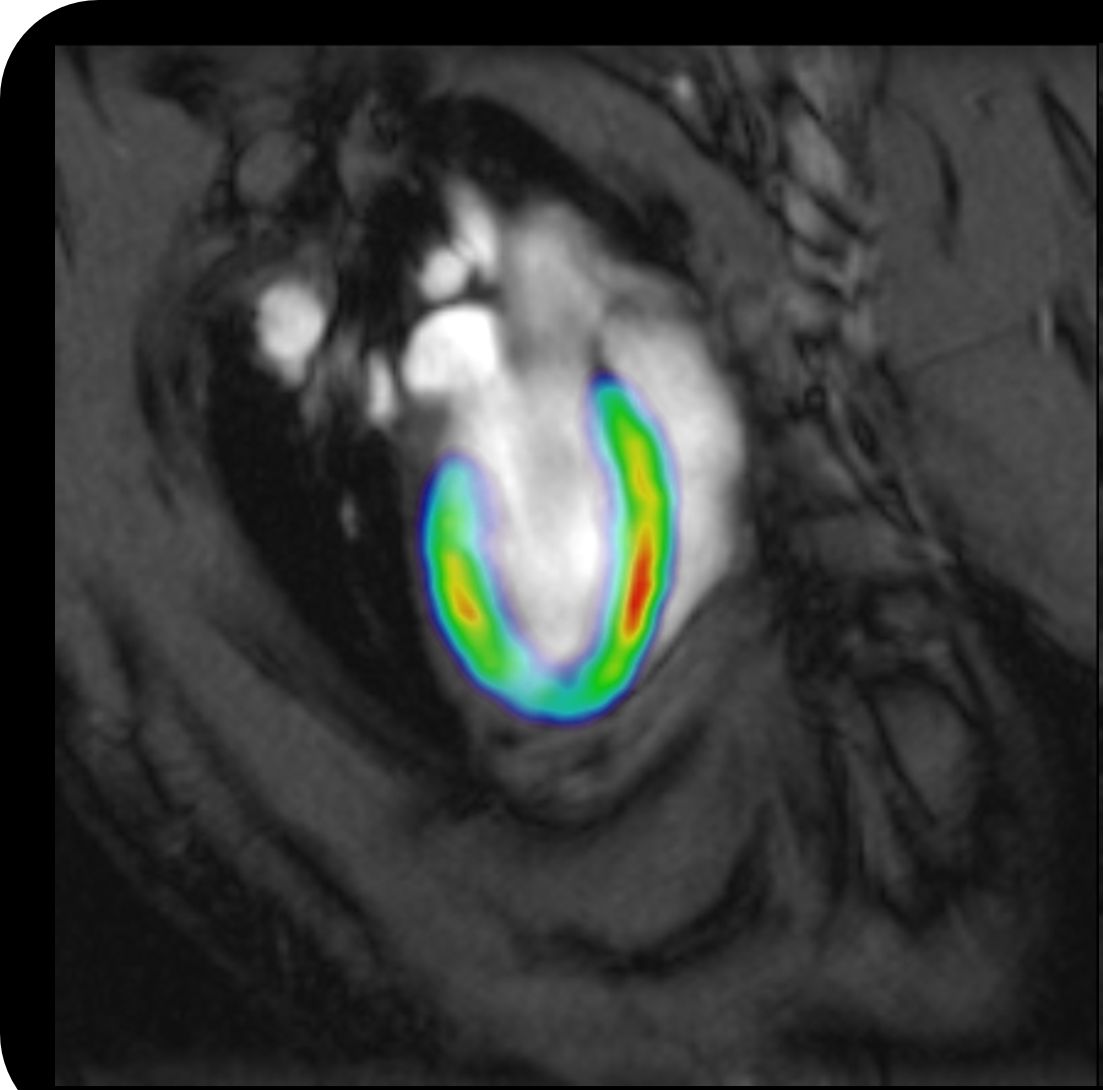
In the clinic, imaging for immunotherapy applications has largely been used to see if the disease worsened, for example, if a new lesion appeared in patients with multiple sclerosis or if metastasis occurred in patients with cancer. That is because the imaging methods available were very insensitive to the molecules and cells responsible for these diseases. On occasion, immune cells would be tracked by "labeling" the cells with metallic agents. This technique has taught us how immune cells move throughout the body to obtain the cues that inform them whether or not to attack. Still, this technique does not tell us what those cues are and what (or how) the immune cells change in response to those cues.
Now, we can monitor more specific markers of immune cell responses using targeted imaging probes. These probes have been used to monitor biological processes associated with inflammation and immune cell activation/deactivation ranging from glucose metabolism to peripheral benzodiazepine receptor expression. Their respective probes, FDG (or fluorodeoxyglucose) and TSPO (or translocator protein), are arguably the most popular molecular imaging biomarkers used to monitor cancer immunotherapies and inflammatory disease progression in clinical trials right now.
And in immunotherapy research?
For preclinical research, we have developed several imaging techniques to monitor the therapy itself, whether it is a small-molecule drug or cell-based. We use these techniques to learn whether the therapy is going where we want, which is to the diseased tissue or to the immune cells, and not to other organs and tissues where side-effects can occur. We have also demonstrated that imaging probes that target the same molecules as candidate therapies can be a powerful tool for predicting if the therapy will be beneficial and for measuring how effective the therapy was. Most of these advances have been for positron emission tomography, an imaging technology that detects the radiation attached to probe or therapy of interest. However, more and more probes are now being developed for or translated for use in magnetic resonance imaging, which will enable us to greatly expand the accessibility of these imaging biomarkers for patients in future.
Your work not only focuses on immunotherapy, but also on biomarkers. Why are these so essential in this context?
My pursuit of imaging biomarkers stems from my research developing therapies. During my graduate studies, I struggled to find imaging biomarkers that could inform me how (well) the immunotherapies I was developing worked in patients. In preclinical research, there are many tools to help scientists gain a better understanding of a disease and the therapies used to treat them, but they cannot be used on patients. This limitation slows down the translation of candidate therapies as well as the selection of approved ones. If we have enough time, we can figure out if a therapy works for a patient. But time is in short supply for patients who can benefit from immunotherapy those with cancer, autoimmune disease, or transplants. Their quality of life can drastically change in the time currently needed to make that decision and by that point, they may not be eligible for alternative or combinatorial treatments that would have worked. On top of that, we rarely know why the therapy doesn't work. It is my belief that the information imaging can provide will streamline medical decisions identify treatments that the patient may benefit from and determine more quickly if a therapy is not effective.
Time is in short supply for patients who can benefit from immunotherapy.
It is my belief that the information imaging can provide will streamline medical decisions.
Dr. Aline Thomas
Dr. Thomas is currently an assistant professor in the Department of Radiology at the Johns Hopkins University School of Medicine (Baltimore, MD). Prior to that, she received two S.B. degrees in Biology and Chemical Engineering at the Massachusetts Institute of Technology (Cambridge, MA) and a Ph.D. in Biomedical Engineering with a specialty in polymer-based biomaterials at Northwestern University (Evanston, IL). She also received postdoctoral training in immunoengineering at the Georgia Institute of Technology (Atlanta, GA) and in molecular MRI at Johns Hopkins University. Her research program uses a combination of biomaterials, computational methods, and molecular imaging to develop novel immunotherapies and translatable imaging biomarkers to evaluate them.