New Research Opportunities at the Brain-Computer Interface: BRAIN Technology Physiology and Non-neuronal Neurocomputation
Electrophysiology and multiphoton technology for in vivo imaging deep within the brain.
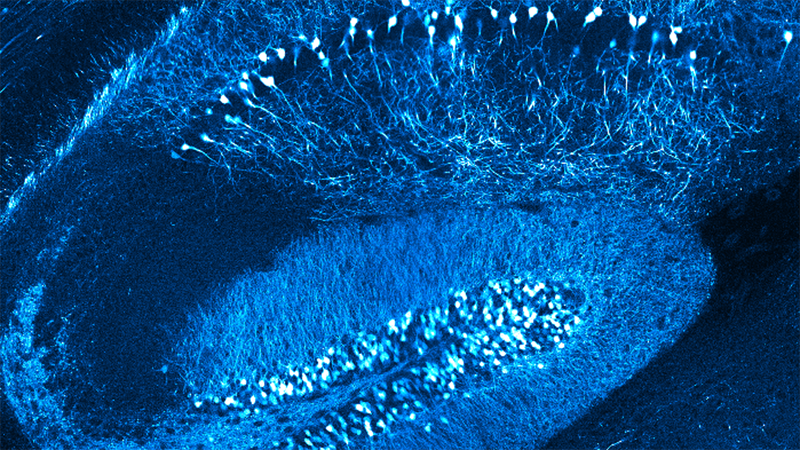
Gain insights into how the combination of electrophysiology, neurotechnologies, and multiphoton imaging create unprecedented data on activity in the brain. Specifically, the dynamics of neurons, microglia, astrocytes, and more.
Research Abstract
Using electrophysiology and engineered neurotechnologies, we track and interrogate neural circuit functions. By utilizing multi-channel electrophysiological arrays and electrochemical assays together with computational signal processing, we map out how neural signals are processed. We combine this method with in vivo multiphoton imaging to tease apart the spatiotemporal dynamics of neurons (quiescent and silenced), microglia, astrocytes, oligodendrocytes, oligodendrocyte progenitor cells, and pericytes. This enables an unprecedented ability to understand the electrical activity of neurons in the context of simultaneous non-neuronal cell activity visualization, which cannot be monitored with traditional electrophysiological technologies.
More recently, we have pivoted to exploring gaps in knowledge regarding electrical stimulation-based activation or inactivation of neural elements over time, which have limited the field’s ability to adequately interpret evoked downstream responses or fine‐tune stimulation parameters to focus on desired effects. Here, we show frequency–dependent differences in spatial and temporal somatic responses during continuous stimulation. In addition, we use genetically encoded activity-dependent fluorescent proteins to track molecular-level activity in specific cell types, such as genetically encoded calcium indicators and glutamate sensors. Our results elucidate conflicting results from prior studies reporting either dense spherical activation of somas biased toward those near the electrode, or sparse activation of somas at a distance via axons near the electrode. Our findings indicate that the neural element-specific temporal response local to the stimulating electrode changes as a function of applied charge density and frequency.
Combined, these results reveal a complex environment around the device-tissue interface and highlight the need for more comprehensive basic science research at the neuroelectronic interface. By developing a better fundamental science understanding at this brain-computer interface, we show that it is possible to intelligently design novel BRAIN Initiative technologies that further enable new modalities for interfacing with the nervous system.
Find out more information about Bruker's solutions for nanoIR Spectroscopy:
Featured Products and Technology
Speaker
Takashi DY Kozai, PhD
Assistant Professor of Bioengineering at the University of Pittsburgh
Takashi Kozai is an Assistant Professor of Bioengineering at the University of Pittsburgh. He received the B.A. (magna cum laude) degree with distinction in Molecular, Cellular, and Developmental Biology, and another B.A. degree with distinction in Biochemistry from the University of Colorado, Boulder, CO, USA, both in 2005, and M.S. and Ph.D. degrees in Biomedical Engineering from the University of Michigan, Ann Arbor, MI, USA, in 2007 and 2011, respectively. From 2011 to 2013, he was a Postdoc with the Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA, where he was appointed as a Research Assistant Professor from 2013-2015 before starting his own lab. His research interests include: (1) Manipulation of neuronal and non-neuronal cells to influence the function of neuronal networks, (2) Understanding the role of neuroimmune cells in neuronal damage and regeneration, and (3) Improving long-term performance of implanted electrodes and integrating man-made (engineered) technology with the human brain for the purpose of studying normal and injured/diseased nervous systems in vivo at the cellular level, as well as restoring function to patients.