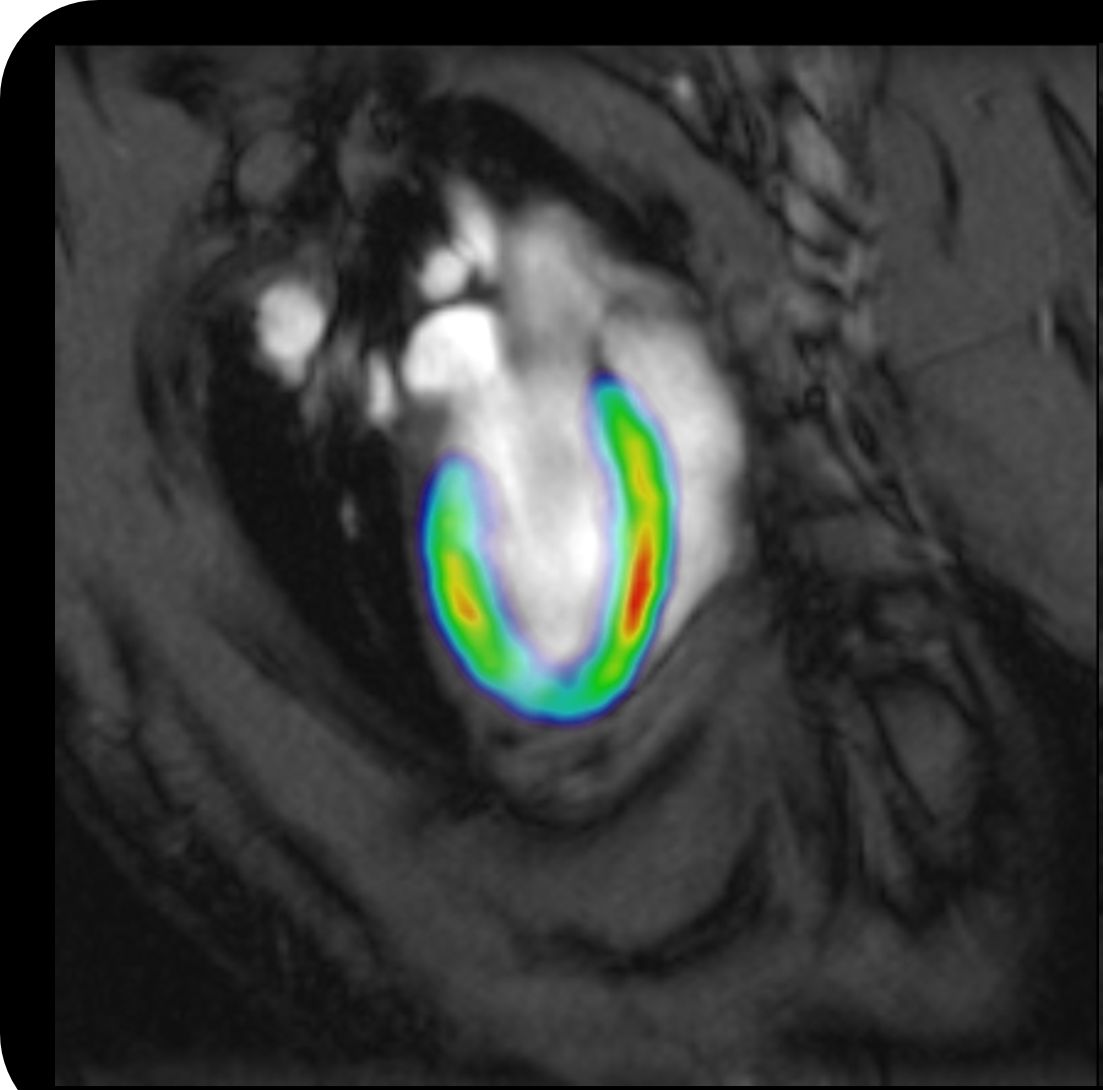
Optogenetic fMRI
Optogenetic fMRI activation areas can be extremely highly targeted. What advantages does this specificity provide?
The highly specific brain activation areas captured by optogenetic fMRI represent downstream targets to which the neural activities initiated at the optogenetically modulated region(s) subsequently propagate. Such specificity is determined by a combination of many factors, mainly including I) viral expression, optogenetic modulation paradigm design, and response properties of the local neuronal populations; II) strength and temporal properties of the initiated neural activities; III) spontaneous activities occuring at multiple scales during the optogenetic perturbation; IV) directness, density and driving/modulatory properties of structural connections between the stimulation site and the downstream targets; V) dynamics of functional connectivity between the stimulation site and the downstream targets, and VI) fMRI data acquisition (i.e., system, setup, sequence, etc) and analyses methods. Using cell-type specific, spatiotemporally-precise, and reversible initiation/manipulation of neural activities while simultaneously visualizing large-scale responses with versatile imaging designs, optogenetic fMRI combines precisely controlled experiments with flexible readout to interrogate the various properties of long-range functional neural circuits/networks and large-scale spatiotemporal coordination of neural activities.
While some neuronal neural networks are quite localized, certain optogenetic stimulations induce brain-wide responses. When can these be seen and what role do they play?
The brain is an extraordinary biological information processing system, consisting of numerous neuronal populations that are connected into functionally specialized circuits and networks. During active tasks or resting, the brain coordinates various neural activities at different spatiotemporal scales across local circuits and/or brain-wide networks to execute functions. Brain-wide responses can be seen with proper stimulations of neuron populations that exhibit brain-wide long-range projections or with the initiation of low frequency activities that are capable of propagating brain-wide. Such brain-wide responses reflect the recruitment of multiple down-stream targets of the neuron populations or neural activities in stimulated sites. They play important roles in dynamic switching of global brain state, selective modulation of brain-wide functional network communication, gating of distributed information storage and organization, etc.
Are there fMRI studies that would be difficult or even impossible to conduct using common stimuli methods?
It is currently impossible to resemble the brain responses of a subject experiencing a multi-sensory context or natural scenery using common optogenetic stimulation methods. With resolution limited by the sub-millimeter scale fiber diameters, it is difficult to perform multisite and impossible to achieve single-cell level precise optogenetic perturbation. Common stimulation paradigm designs utilizing repetitive square pulse stimulation also limit the optogenetic fMRI studies in examining more physiological responses that are elicited in natural situations. Meanwhile, close-loop stimulation of neuronal populations require real-time monitoring of local neuronal activities with millisecond-scale temporal resolution to determine the dynamic states of local neuronal populations or networks, which is difficult to conduct using common stimulation methods.
Optogenetic fMRI is predominately a preclinical technology. How do findings with this methodology translate into clinical relevance?
Findings in optogenetic fMRI studies in healthy controls can improve our understanding of how the normal brain works and facilitate the development of fMRI/neuroimaging methodology which will be adopted in clinical settings. Meanwhile, the differences in optogenetic fMRI results between normal and disease models can guide the development of diagnostic techniques and elucidate underlying mechanisms for diseases. Further, optogenetic fMRI findings in disease models can be used to guide the development of therapeutic interventions.
How far are we away from understanding neural networks on a cellular level?
Currently, fMRI cannot achieve cellular-level spatial resolution, however, several aspects of technological development could aid us in examining neural networks on a cellular level in the coming years. First, cellular-level manipulations of neural circuits with versatile cutting-edge genetic tools in combination with fMRI have begun to provide us with great opportunities to examine the outcome of perturbing neural networks on a cellular level. Second, the combination of cellular-level resolution optical imaging with optogenetic fMRI will help deepen our understanding of neural networks on a cellular level. Third, with the recent and ongoing development of precision optogenetic stimulation methods (such as holographic stimulation), we will be able to conduct optogenetic stimulation on a cellular level while visualizing the consequential effects with whole brain fMRI imaging.
The brain is an extraordinary biological information processing system.
We will be able to conduct optogenetic stimulation on a cellular level while visualizing the consequential effects with whole brain fMRI imaging.
Prof. Ed X. Wu
Dr. Wu is the Lam Woo Professor and Chair of Biomedical Engineering at the University of Hong Kong. He obtained his BEng in Electrical Engineering from Tianjin University in 1984, MSc in Medical Physics from University of Wisconsin - Madison in 1988, and PhD in Radiological Sciences from University of California - Irvine in 1993. From 1990 to 2003, Dr. Wu worked in Columbia University in New York City first as an Assistant Professor and later as an Associate Professor of Radiology and Biomedical Engineering. Dr. Wu moved to University of Hong Kong in 2003. His research interest encompasses MRI biophysics, reconstruction algorithms, advanced biomedical applications, and system engineering for accessible healthcare (www4.hku.hk/bisplab/). At present, one of his key research focuses is to develop state-of-the-art functional MRI methodologies for probing brain circuits and functions in rodent models by combing functional, diffusion and spectroscopic MRI with electrophysiology, behavioral assessment and neuromodulation approaches such as optogenetics. Dr. Wu is an elected Fellow of ISMRM, IEEE, and AIMBE. Dr. Wu is the Asia-Pacific Editor of NMR in Biomedicine (NBM) since 2011.